
Waterproofing Services: The Unsung Protectors of Our Buildings
Often, when we admire a building, our eyes are drawn to its architectural beauty, … Read more
The Real Estate Agent: Navigating the Property Tapestry with Expertise
In the vast and intricate world of real estate, properties stand as more than … Read more

Unpacking the Value of Moving Companies in Successful Relocations
Relocating to a new home or office is an exhilarating adventure. A fresh start, … Read more

The Unsung Heroes of Motherhood: Celebrating Lactation Consultants
Motherhood, with all its beauty, also brings forth challenges, many of which are unforeseen … Read more

The Culinary Choreography of Catering Services
In the theatre of events, there’s an unspoken star that often captivates the audience’s … Read more

St. Catharines Metal Supplier: Molding the Metallic Essence of the Garden City
St. Catharines, the jewel of Niagara, is a city that seamlessly weaves natural splendour … Read more

Opticals: More Than Just a Fashion Statement
The world of optical is vast and multifaceted. From vision correction to making bold … Read more

Milwaukee Packout: The Ultimate Modular Storage Solution
When it comes to organizing tools and ensuring they’re easily transportable, the Milwaukee Packout … Read more
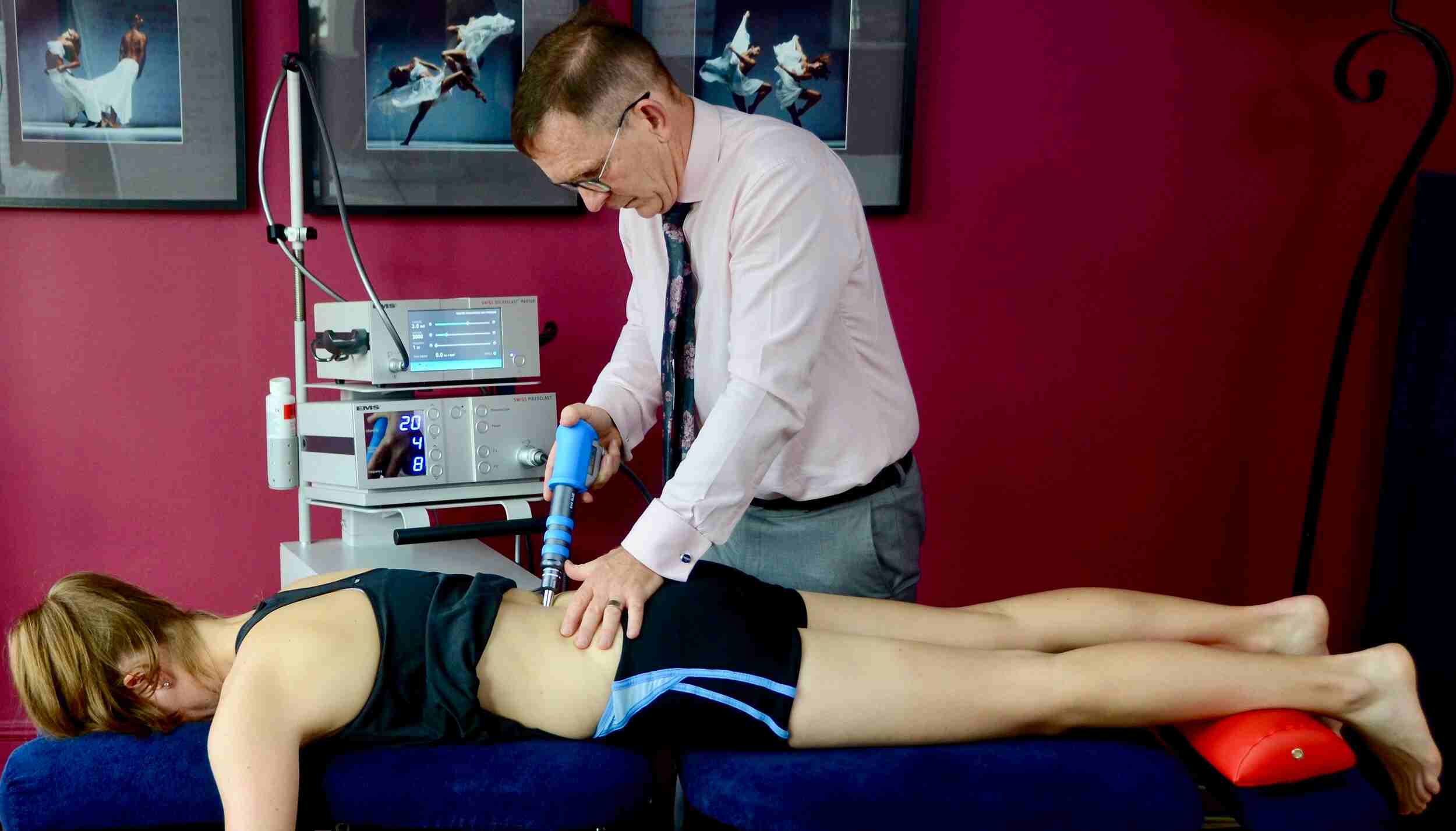
Shockwave Therapy: A Modern Solution to Chronic Pain
In the last few decades, medical science has made leaps and bounds in developing … Read more
Receive the latest articles in your inbox
Insert your email signup form below
[insert e-mail subscription form]